Abstract
Background BCR-ABL1 T315I, "gatekeeper," mutation can confer a high degree of resistance to many first- and second-generation TKIs. Olverembatinib is a novel, orally active, third-generation BCR-ABL1 TKI with promising efficacy and a preliminary favorable safety profile for the treatment of patients with CML. These phase 2 trials, HQP1351-CC-201 (NCT03883087) and
HQP1351-CC-202 (NCT03883100), were conducted based on favorable phase 1 trial results.
Methods These open-label, single-arm, multicenter pivotal trials evaluated the efficacy and safety of olverembatinib in Chinese pts with CML-CP (HQP1351-CC-201) or CML-AP
(HQP1351-CC-202) with the T315I mutation. Olverembatinib was administered at 40 mg orally on alternate days (QOD) for 28-day cycles. Primary endpoints were major cytogenetic response (MCyR) in pts with CML-CP and major hematologic response (MaHR) in those with CML-AP by the end of Cycle 12. Secondary study endpoints included complete CyR (CCyR), complete hematologic response (CHR), major molecular response (MMR), progression-free survival (PFS), overall survival (OS), and safety, including treatment-related adverse events (TRAEs) and serious AEs (SAEs).
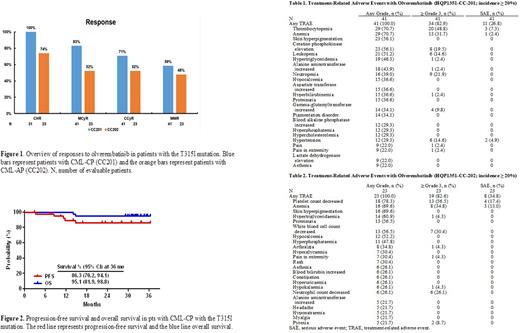
ResultsHQP1351-CC-201 As of the cutoff date of April 30,2022, 41 pts were enrolled, of whom 21 (51.2%) were male, with a median (range) age of 47 (22-70) years. The median (range) interval from CML diagnosis to first olverembatinib dose was 5.31 (0.6-23.2) years, and 32 (78.1%) pts had received ≥ 2 prior TKIs. The median (range) treatment duration was 32.7 (3.1-36.7) months. All pts, 31/31 (100%), achieved CHR (10 others had CHR at baseline), 34/41 (82.9%) MCyR, 29/41 (70.7%) CCyR, and 24/41 (58.5%) MMR (Figure 1). Median time to CHR was 1 (95% CI: 1.0-1.9) month, median time to MCyR was 2.8 (95% CI: 2.8-5.6) months, and median time to MMR was 6.5 (95% CI: 2.8 to not reached [NR]) months. At 36 months, the PFS rate was 86.3% (95% CI: 70.2%-94.1%) and the OS rate was 95.1% (95% CI: 81.9%-98.8%) (Figure 2). A total of 5 pts withdrew because of progressive disease (PD), 4 intolerance, 3 consent withdrawal, and 2 for other reasons. Frequent TRAEs (all grades; grade 3-4; SAEs) included thrombocytopenia (70.7%; 48.8%; 7.3%), anemia (70.7%; 31.7%; 2.4%), leukopenia (51.2%; 14.6%; 0), and neutropenia (41.4%; 21.9%; 0). Common nonhematologic TRAEs (all grades; grade 3-4) included skin pigmentation (56.1%; 0%) and elevations in creatine kinase (56.1%; 19.5%), ALT (43.9%; 2.4%) and AST (36.6%; 0) levels (Table 1).
HQP1351-CC-202 As of the cutoff date of April 30,2022, 23 pts were enrolled, of whom 18 (78.3%) were male, with a median (range) age of 41 (21-74) years. The median (range) interval from CML diagnosis to first olverembatinib dose was 4.96 (0.4-10.2) years, and 19 (82.6%) pts had received ≥ 2 prior TKIs. The median (range) treatment duration was 19.7 (1.4-36.4) months. A total of 18 (78.3%) patients experienced MaHR (73.9% CHR and 4.4% no evidence of leukemia [NEL]); 12 (52.2%) MCyR; 12 (52.2%) CCyR; and 11 (47.8%) MMR (Figure 1). Median time to MaHR was 2.8 (95% CI: 1.0-4.7) months, median time to MCyR was 5.6 (95% CI: 2.00-NR) months, and median time to MMR was 13.1 (95% CI: 5.6-22.4) months. At 36 months, the PFS rate was 57.1% (95% CI: 33.3%-75.1%) and the OS rate was 69.6% (95% CI: 46.6%-84.2%) (Figure 3). Six pts withdrew because of progressive disease (PD), 4 intolerance, and 1 for other reasons; two pts died. Common TRAEs (all grades; grade 3-4; SAEs) included thrombocytopenia (78.3%; 56.5%; 17.4%), anemia (69.6%; 34.8%; 13.0%), leukopenia (56.5%; 30.4%; 0), and neutropenia (26.1%; 26.1%; 0). Common nonhematologic AEs included skin pigmentation (69.6%), hypocalcemia (52.2%), proteinuria (56.5%), hypertriglyceridemia (60.9%), hyperphosphatemia (47.8%), hyperuricemia (26.1%), and arthralgia (34.8%), of which most were grade 1-2 (Table 2).
Conclusions Olverembatinib was efficacious and well tolerated in pts with TKI-resistant
CML-CP and CML-AP with the BCR-ABL1T315I mutation. Based on the results of these pivotal trials, the Chinese health authority granted conditional approval for olverembatinib on November 24, 2021.
Disclosures
Chen:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Niu:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Men:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Yang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership, Patents & Royalties. Zhai:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal